Hydrogen embrittlement is a physical-chemical-metallurgical phenomenon. It is developed by chemical interaction and diffusion in the metal. Depending on the metallurgical nature of the substrate where it takes place may cause critical changes in the capacity of load transmission of the elements under stress. This can lead to catastrophic fracture at lower loads which supports the material under normal conditions.
Even if this phenomenon takes place preferentially in carbon or low-alloy, other metals and alloys are susceptible to this kind of damage. The hydrogen embrittlement phenomena, in whatever their form of expression, severely restricts the applicability of certain materials.
The interaction between hydrogen and metals, can lead to the formation in the metal of solid solution of hydrogen, molecular hydrogen and gaseous products formed by the reaction between hydrogen and the constitutive metals of the alloy.
Depending on the type of interaction between the hydrogen and the metal, hydrogen damage can appear in different ways, all of them harmful from the mechanical behavior point of view.
The source of hydrogen in the structure of a component may be linked with the manufacturing processes of the raw material as well as its subsequent processing, or may be incorporated into the material during its service life.
Most of low-temperature corrosion processes are caused by aqueous or wet corrosion. Direct oxidation is much less important in these conditions. Wet corrosion has electrochemical origin and is not uniform on the surface of the metal.
Corrosion phenomena results from the formation of electric batteries, generally microscopic, formed between different but not identical parts of a metal. These microcells cause a local electric current and lead to the existence of a flow of electrons through the metal between the area where the oxidation takes place (anode) and the reduction area (cathode). Both areas can be separated from interatomic distances up to kilometers.
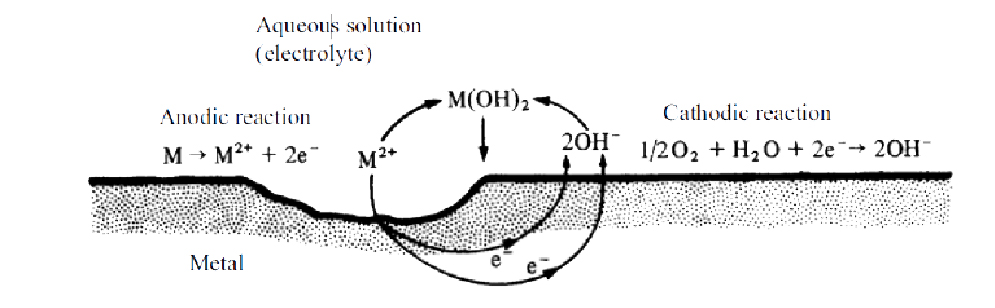
IK4-AZTERLAN is developing specific work lines to evidence the mechanisms of hydrogen embrittlement of certain grades of steel.
The two introduction ways of hydrogen in the metal studied at this moment are:
– The contact between the metallic material and one electrolyte able to provide hydrogen
-The cathodic protection
Even if some tests are already standardized for certain components, IK4-AZTERLAN has identified the optimal conditions to evidence this failure mode. At the same time, IK4-AZTERLAN has defined specific variables helping in the evaluation of its incidence.
The characterizations allowing this control are:
– Tensile tests with low strain rate in corrosive environment. This complex characterization allows to measure the effect of the environment on the materials through parameters related to the loss of ductility.

– Tensile tests at constant load in corrosive environment. This method evaluates the occurrence of cracks or breakage of specimens.
Este método evalúa la aparición de grietas o rotura de probetas.

Both methods are comparatives and seek to establish limitations on the use of certain environments and conditions to avoid hydrogen embrittlement.


